Rapid testing has emerged as a critical tool in bolstering flu surveillance efforts, providing a nimble and efficient means of tracking and managing the spread of influenza. In the realm of infectious diseases, time is of the essence, and traditional testing methods often fall short in delivering timely results. Rapid testing, on the other hand, brings a paradigm shift by offering quick and accurate diagnoses, enabling health authorities to respond swiftly to potential outbreaks. One of the key realities of rapid testing in the context of flu surveillance is its ability to bridge the gap between symptom onset and confirmation. Unlike conventional laboratory tests that may take days to yield results, rapid tests deliver outcomes within minutes, allowing for prompt identification of influenza cases. This speed is instrumental in implementing targeted public health measures, such as isolation protocols and contact tracing, to contain the spread of the virus effectively.
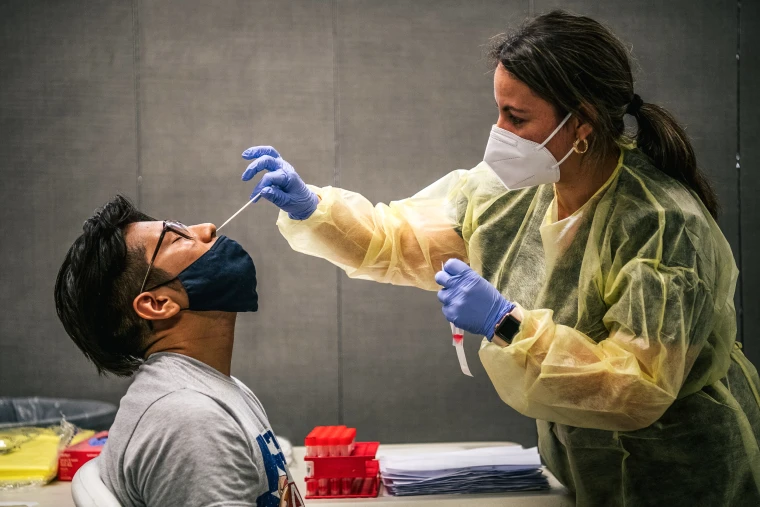
Moreover, rapid testing enhances the granularity of flu surveillance data. By swiftly identifying and confirming cases, health agencies can collect real-time information on the prevalence and geographic distribution of influenza and visit us. This granularity is invaluable in designing targeted vaccination campaigns and allocating medical resources where they are most needed. The ability to adapt swiftly to evolving patterns of influenza transmission is a critical advantage in the ongoing battle against respiratory viruses. Another reality is the accessibility and ease of deployment of rapid testing technologies. These tests can be administered in diverse settings, from healthcare facilities to community centers, and even remote areas with limited access to traditional laboratory infrastructure. This democratization of testing empowers a broader spectrum of healthcare providers and communities to actively participate in flu surveillance efforts, fostering a more comprehensive and decentralized approach to monitoring and managing influenza outbreaks.
However, challenges exist in the widespread adoption of rapid testing for flu surveillance. Issues related to test accuracy, especially in the early stages of infection, and variations in the sensitivity of different tests must be addressed. Furthermore, the affordability and availability of rapid testing kits pose challenges, particularly in resource-limited regions. Overcoming these obstacles requires concerted efforts from governments, healthcare institutions, and the private sector to ensure equitable access to reliable and cost-effective rapid testing solutions. In conclusion, rapid testing has proven to be a transformative force in enhancing flu surveillance efforts. Its ability to provide timely results, offer granularity in data collection, and be deployed in diverse settings contributes significantly to the proactive management of influenza outbreaks. As technology continues to advance and the global community unites in the fight against infectious diseases, the integration of rapid testing into routine flu surveillance strategies holds the promise of a more resilient and responsive public health infrastructure.
